2道大学化学题,1.which of the following molecules has the lowest bo
来源:学生作业帮 编辑:神马作文网作业帮 分类:化学作业 时间:2024/11/17 12:19:37
2道大学化学题,
1.which of the following molecules has the lowest boiling point?
A.CH4 B.NH3 C.SiH4 D.HF E.H2O
这里我不理解怎样判断沸点的高低,请解释如何判断,
2.which of the following subtances will participate in hydrogen bonding in the liquid phase.
A.CH4 B.NaH C.SiH4 D.CH3(CO)H E.CH3OH
我这里有答案,但我不理解为什么
1.which of the following molecules has the lowest boiling point?
A.CH4 B.NH3 C.SiH4 D.HF E.H2O
这里我不理解怎样判断沸点的高低,请解释如何判断,
2.which of the following subtances will participate in hydrogen bonding in the liquid phase.
A.CH4 B.NaH C.SiH4 D.CH3(CO)H E.CH3OH
我这里有答案,但我不理解为什么
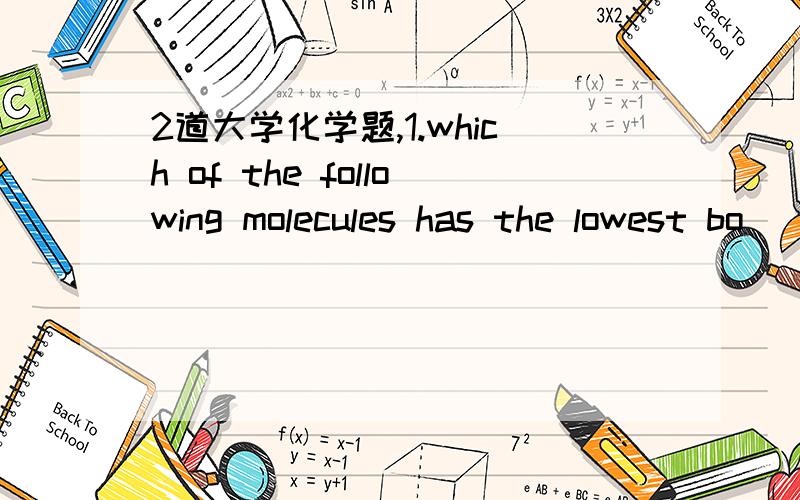
第一题A,主要从分子间作用力与氢键判断,可以形成氢键的有F ,O,CI,N,选项BDE都可以形成氢键,原子氧化性越强,形成的氢键越强,故D>B,对于E,两分子H2o形成的氢键数目多于两分子HF形成的氢键数目(氢键是分子间形成的作用力,而不是分子内部的作用力,就是一个单独的水分子只有没有氢键的存在),故E>D
A与C选项主要看分子间作用力,分子量越大,分子间作用力越强,沸点越高,故C>A
第二题选E,任然是说形成氢键的原子F,O,Cl,N,即A,B,C就不涉及氢键,对于D中的氧原子,它只有两个单键,并且全部与C的两个单键结合,因此就没有空出的键区结合另一个分子中氢,E可以看作是一个水分子中氢被甲基取代,故于水内似可以形成氢键,只不过形成氢键数目减少
再问: 第二题是E
再答: 知道了,hydrogen bonding,这个翻译错了,呵呵
A与C选项主要看分子间作用力,分子量越大,分子间作用力越强,沸点越高,故C>A
第二题选E,任然是说形成氢键的原子F,O,Cl,N,即A,B,C就不涉及氢键,对于D中的氧原子,它只有两个单键,并且全部与C的两个单键结合,因此就没有空出的键区结合另一个分子中氢,E可以看作是一个水分子中氢被甲基取代,故于水内似可以形成氢键,只不过形成氢键数目减少
再问: 第二题是E
再答: 知道了,hydrogen bonding,这个翻译错了,呵呵
一道大学普通化学题Which of the following is non-SI unit——milliliter,m
一道SAT2化学题Which of the following electron configurations repr
Which one of the following materials has the highest modulus
英语翻译1.Which of the following sets of whole numbers has the l
Which of the following sentences has an object complement?A.
GMAT which of the following fractions has a decimal equivale
Which of the following words has two syllables?A
Which of the following sentences has an object complement?
Which of the following has the greatest value?A.1.73^999 B.2
Which of the following is
which of the following意思?
又一道英文化学题..Which one of the following is not the same number